Ex.18 In the system, LaCl3(s) + H2O(g) + Heat = LaCIO(s) + 2HCl(g), is established. More water vapour is added to reestablish the equilibrium. The pressure of water vapour is doubled. The
Par un écrivain mystérieux
Last updated 17 juin 2024

Click here:point_up_2:to get an answer to your question :writing_hand:ex18in the systemlacl3s h2og heat lacios 2hclgis established more water vapour is
Click here👆to get an answer to your question ✍️ Ex-18 In the system- LaCl3-s- - H2O-g- - Heat - LaCIO-s- - 2HCl-g- is established- More water vapour is added to reestablish the equilibrium- The pressure of water vapour is doubled- The factor by which pressure of HCl is changed is - -A-2 -B- V -C- 13 -D
Click here👆to get an answer to your question ✍️ Ex-18 In the system- LaCl3-s- - H2O-g- - Heat - LaCIO-s- - 2HCl-g- is established- More water vapour is added to reestablish the equilibrium- The pressure of water vapour is doubled- The factor by which pressure of HCl is changed is - -A-2 -B- V -C- 13 -D
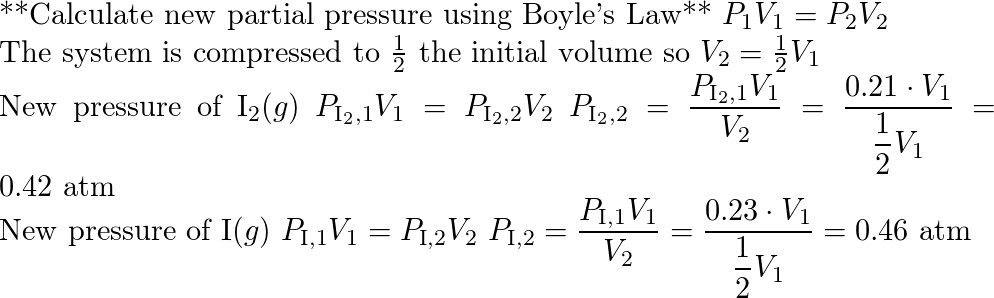
A system at equilibrium contain s $l_2(g)$ at a pressure of

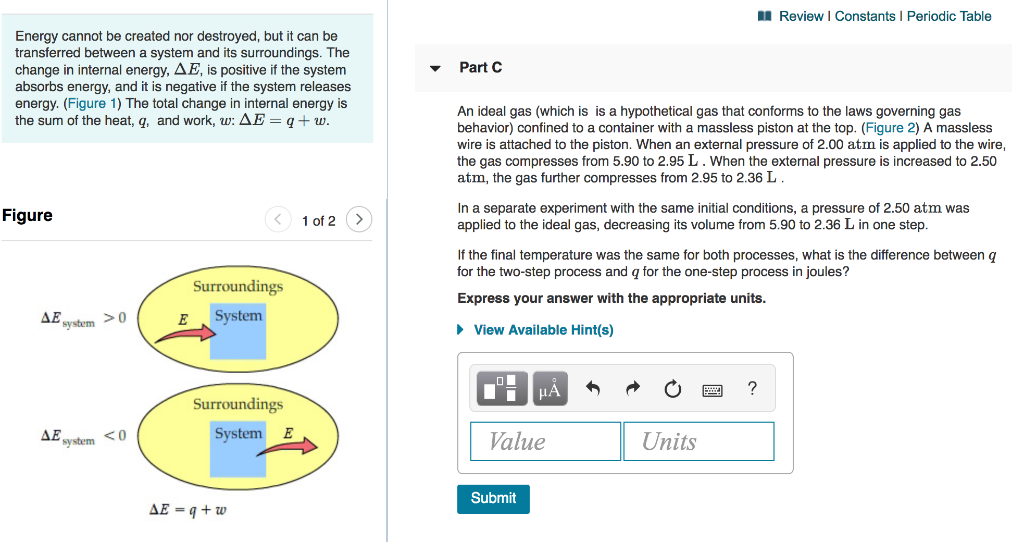
Solved b. An ideal gaseous reaction (which is a hypothetical
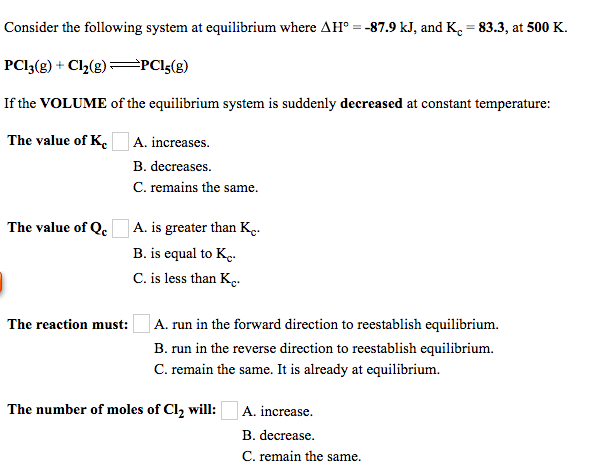
Solved Consider the following system at equilibrium where

Chemistry 2 Flashcards
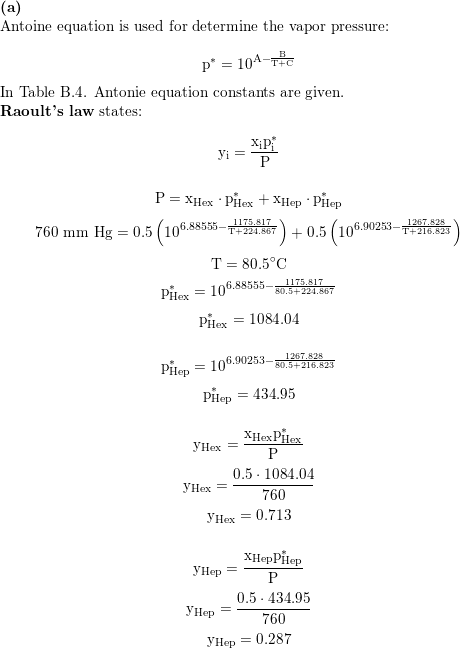
Calculate the following: a) The bubble-point temperature of
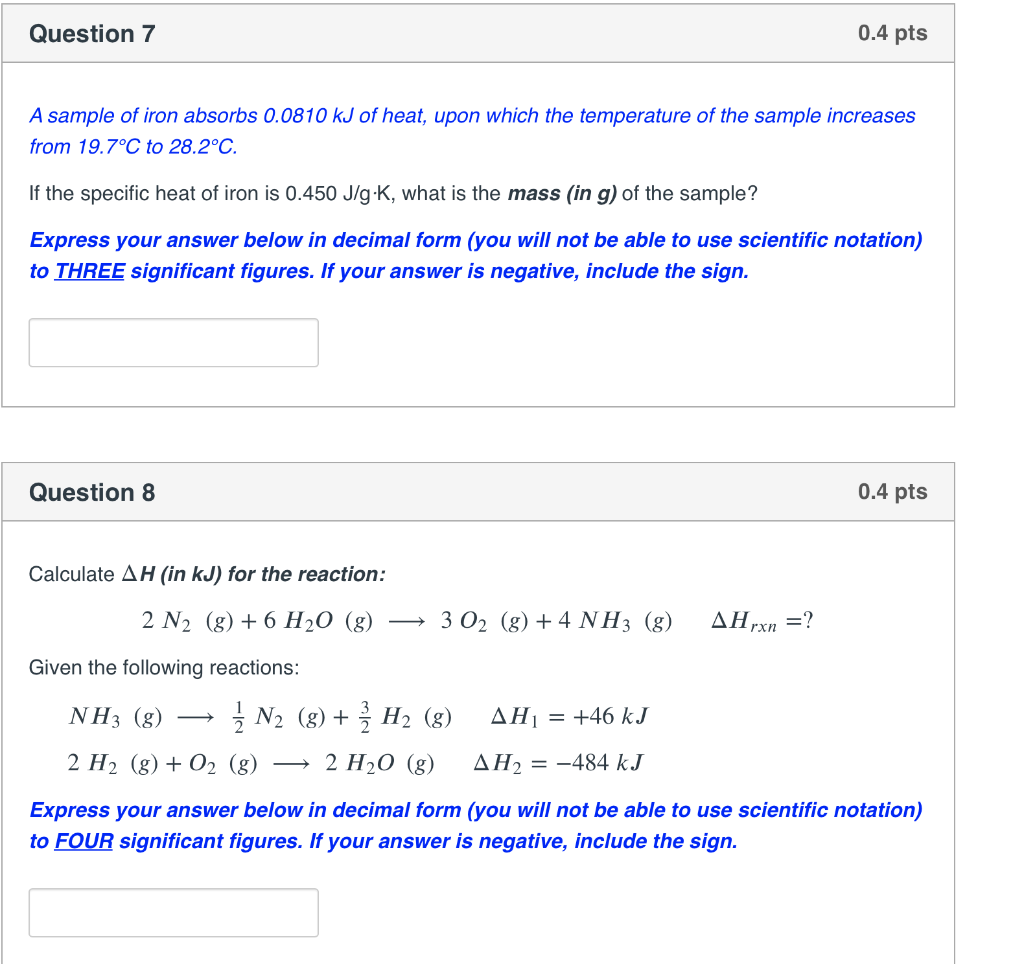
Solved Question 3 0.4 pts How much work (in J) does a gas
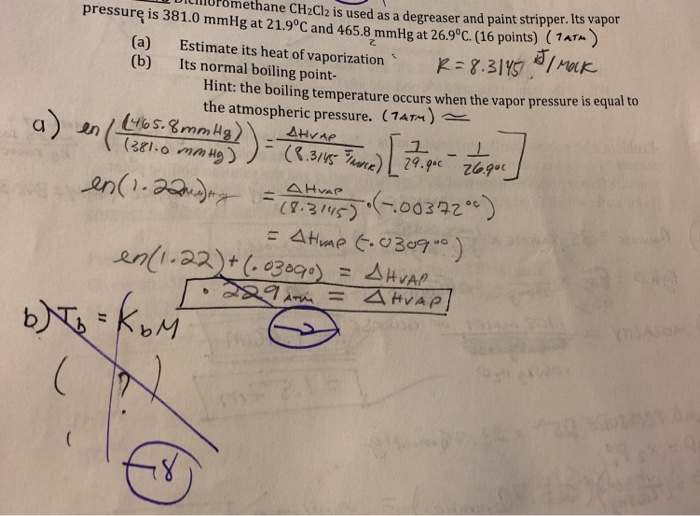
Solved 1. Calculate the heat release by cooling 55.0g H2O

Solved 1. Consider the following partial pressures of HCl
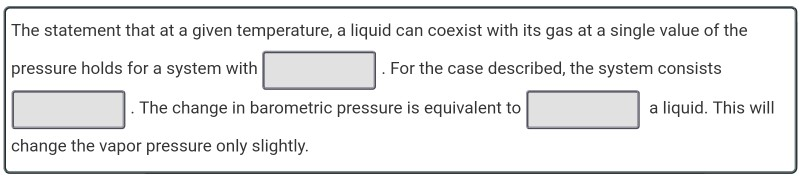
Solved At a given temperature, a liquid can coexist with its
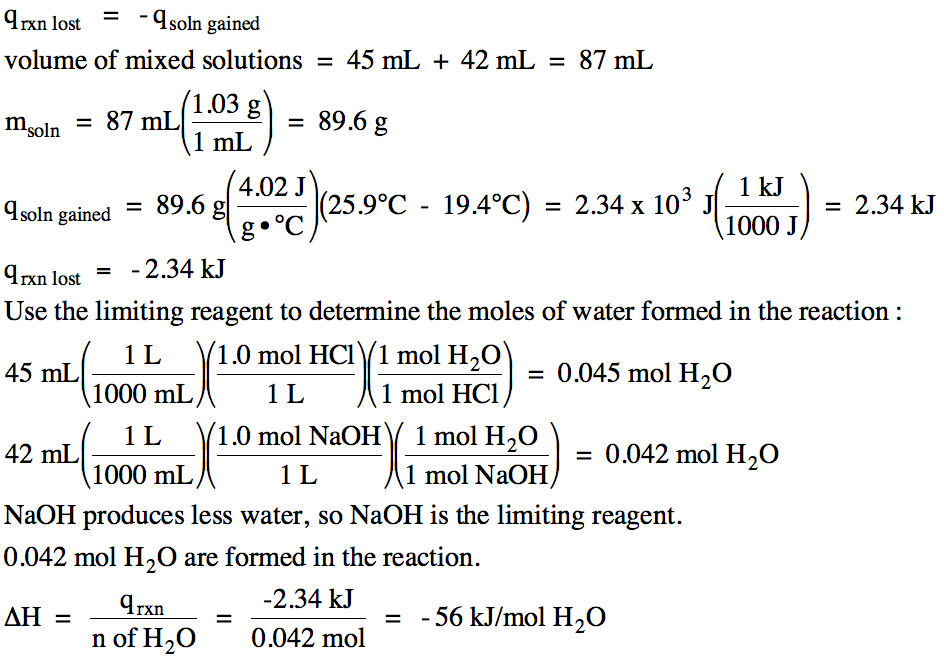
image035.png
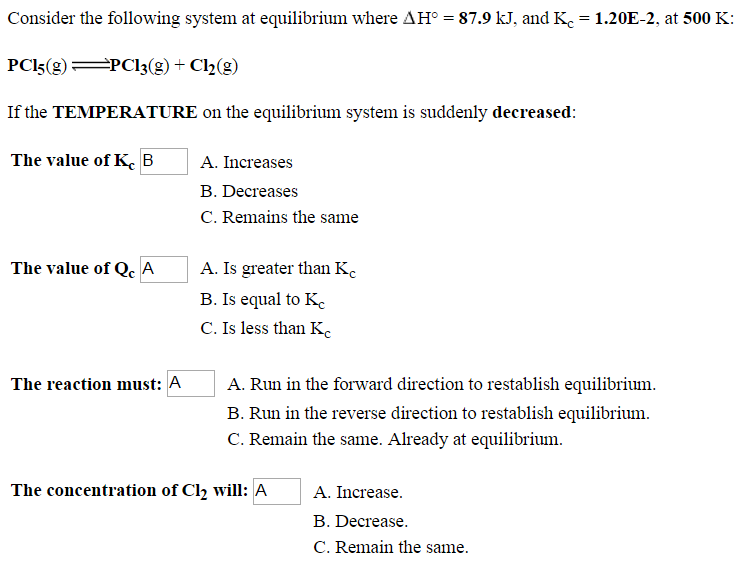
Solved Consider the following system at equilibrium where
Recommandé pour vous
 Gants chauffants fins + Batterie - G-HEAT - Loisir-Plein-Air14 Jul 2023
Gants chauffants fins + Batterie - G-HEAT - Loisir-Plein-Air14 Jul 2023 Gilet chauffant professionnel Vêtement chauffant G-Heat Pro14 Jul 2023
Gilet chauffant professionnel Vêtement chauffant G-Heat Pro14 Jul 2023 G-Heat présente sa collection hiver 2019/2020 – : Pour tout savoir sur la marche – Site et Webzine sur la marche : Marche Nordique / Marche Athlétique / Randonnée Pédestre /14 Jul 2023
G-Heat présente sa collection hiver 2019/2020 – : Pour tout savoir sur la marche – Site et Webzine sur la marche : Marche Nordique / Marche Athlétique / Randonnée Pédestre /14 Jul 2023- G-Heat - Bonsoir à tous, Cette courte publication pour vous indiquer qu'un site internet denommé www.h-heat.com a presque copié notre url le mois dernier et vous êtes environ une centaine à avoir14 Jul 2023
 G Super Heat Liquid, Packaging Type: pet bottle, 210ml at Rs 85/kg in Roorkee14 Jul 2023
G Super Heat Liquid, Packaging Type: pet bottle, 210ml at Rs 85/kg in Roorkee14 Jul 2023 Gilet rafraichissant G-Heat EV05-GRE14 Jul 2023
Gilet rafraichissant G-Heat EV05-GRE14 Jul 2023 Gants de ski chauffants en cuir EVO-3 + Batterie - G-HEAT - Pecheur-Online14 Jul 2023
Gants de ski chauffants en cuir EVO-3 + Batterie - G-HEAT - Pecheur-Online14 Jul 2023 G)I-DLE - HEAT (Premium Sleeve FLARE Ver.)14 Jul 2023
G)I-DLE - HEAT (Premium Sleeve FLARE Ver.)14 Jul 2023 Sweat à capuche noir chauffant G-HEAT14 Jul 2023
Sweat à capuche noir chauffant G-HEAT14 Jul 2023 G-Heat dévoile ses dernières innovations pour l'hiver - votre magazine vélo et triathlon14 Jul 2023
G-Heat dévoile ses dernières innovations pour l'hiver - votre magazine vélo et triathlon14 Jul 2023
Tu pourrais aussi aimer
 TOPDON Topscan Bluetooth OBD2 Car Code Reader All System ABS SRS Scan Tool US14 Jul 2023
TOPDON Topscan Bluetooth OBD2 Car Code Reader All System ABS SRS Scan Tool US14 Jul 2023 DUSTERTEAM • Afficher le sujet - Support telephone RAM14 Jul 2023
DUSTERTEAM • Afficher le sujet - Support telephone RAM14 Jul 2023 Les fleurs comestibles14 Jul 2023
Les fleurs comestibles14 Jul 2023 Cyclisme Vélo Vélo Tête Tube Guidon Téléphone Portable Sac Étui14 Jul 2023
Cyclisme Vélo Vélo Tête Tube Guidon Téléphone Portable Sac Étui14 Jul 2023- Répertoire Alphabétique spirale Clairefontaine Metric 9,5 x 14 cm14 Jul 2023
 Lait de croissance 2ème âge14 Jul 2023
Lait de croissance 2ème âge14 Jul 2023 Playmobil 1.2.3. - Pousser et partir Voiture - 2 Parties - 7132314 Jul 2023
Playmobil 1.2.3. - Pousser et partir Voiture - 2 Parties - 7132314 Jul 2023 Kinder Schoko-bons crispy - 92 g14 Jul 2023
Kinder Schoko-bons crispy - 92 g14 Jul 2023 Balance de précision 0.01g - Balance Professionnelle14 Jul 2023
Balance de précision 0.01g - Balance Professionnelle14 Jul 2023 haute-loire - Humanitaire. Cap sur Marrakech pour Caroline Sauvage14 Jul 2023
haute-loire - Humanitaire. Cap sur Marrakech pour Caroline Sauvage14 Jul 2023
