AAFP Backs New EUAs on COVID-19 Boosters for Children
Par un écrivain mystérieux
Last updated 15 juin 2024

The AAFP reviewed evidence on the use of bivalent COVID-19 booster vaccines in children and adolescents, and approved action by the FDA and CDC on amended emergency use authorizations.

COVID-19 Vaccine Updates

COVID-19 Vaccine Information - Tacoma-Pierce County Health Department

COVID vaccines for kids will be in high demand, experts urge patience

Children Ages 6 Months Through 4 Years Now Eligible for COVID-19 Vaccine
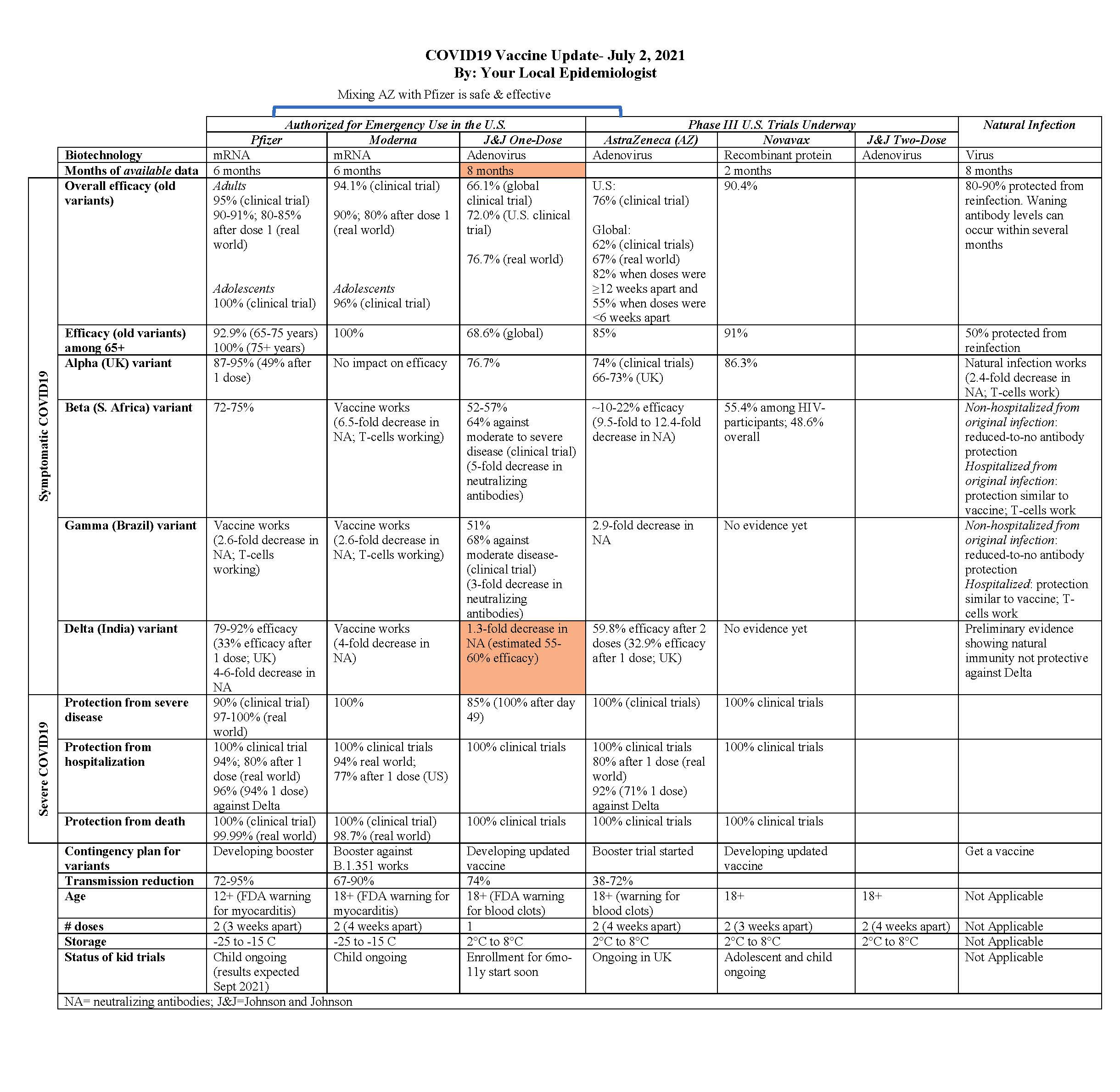
All Chapters - COVID-19 Protocols

AAFP Supports Expanded EUAs on Bivalent COVID-19 Boosters

AAFP Approves Federal Actions on COVID-19 Boosters

Birth-18 Years Immunization Schedule, By Medical Condition

FDA authorizes updated COVID booster shots for kids as young as 5 - CBS Boston
Recommandé pour vous
 The 4 Best Booster Car Seats of 202414 Jul 2023
The 4 Best Booster Car Seats of 202414 Jul 2023 File:MBK Booster, yellow.jpg - Wikimedia Commons14 Jul 2023
File:MBK Booster, yellow.jpg - Wikimedia Commons14 Jul 2023- Graco TurboBooster Highback LX Booster Car Seat with Safety Surround - Stark14 Jul 2023
 BOOSTER14 Jul 2023
BOOSTER14 Jul 2023 CDC recommends new COVID booster for all Americans over 6 months amid rising cases, hospitalizations - ABC News14 Jul 2023
CDC recommends new COVID booster for all Americans over 6 months amid rising cases, hospitalizations - ABC News14 Jul 2023- Nature's Bounty Advanced Metabolism Booster, 120 Capsules14 Jul 2023
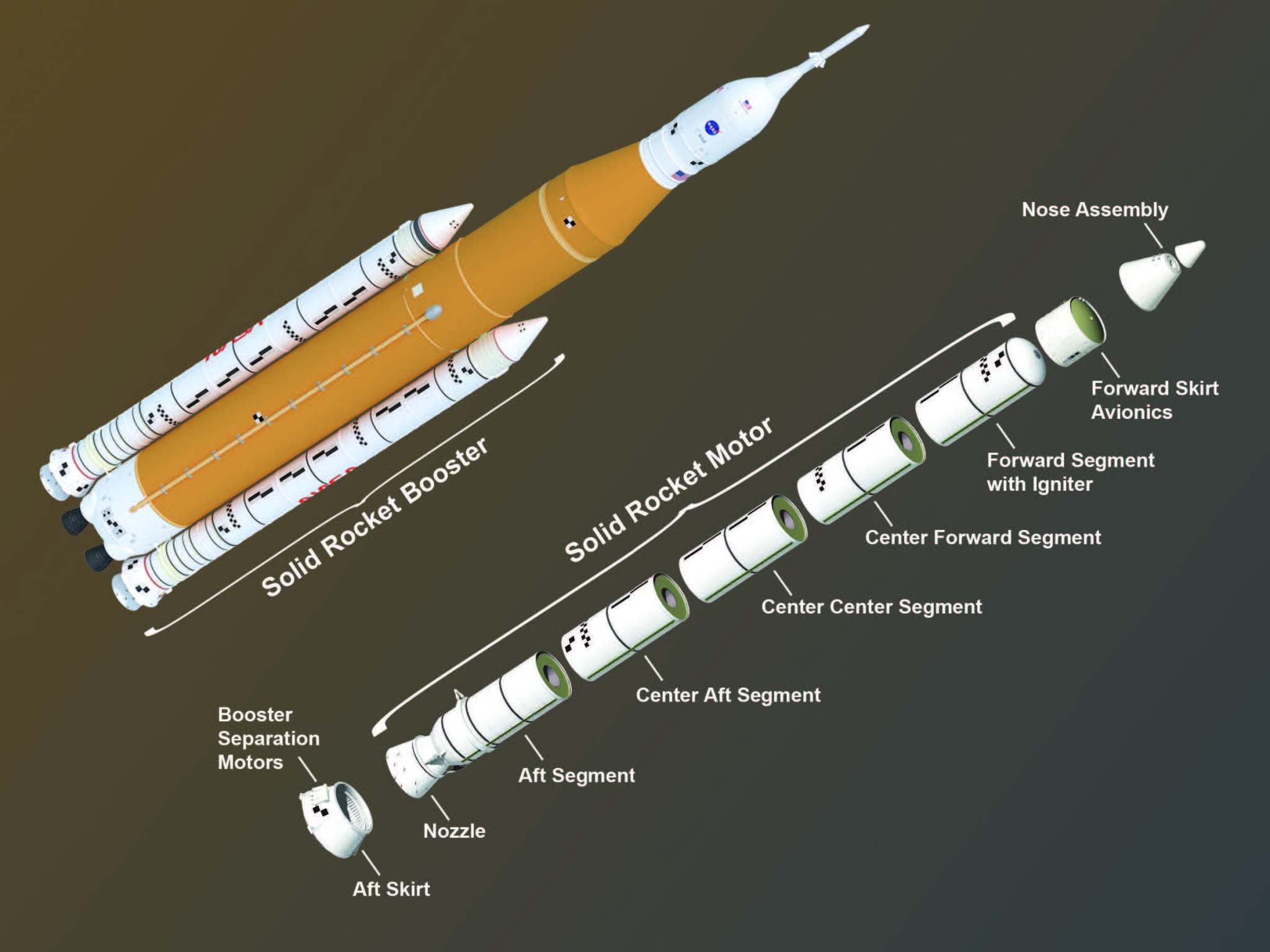 Space Launch System Solid Rocket Booster - NASA14 Jul 2023
Space Launch System Solid Rocket Booster - NASA14 Jul 2023 Do I Need a COVID-19 Booster Shot? 6 Questions Answered on How to Stay Protected14 Jul 2023
Do I Need a COVID-19 Booster Shot? 6 Questions Answered on How to Stay Protected14 Jul 2023 Pokémon TCG: Sword & Shield-Brilliant Stars Booster Display Box (36 Packs)14 Jul 2023
Pokémon TCG: Sword & Shield-Brilliant Stars Booster Display Box (36 Packs)14 Jul 2023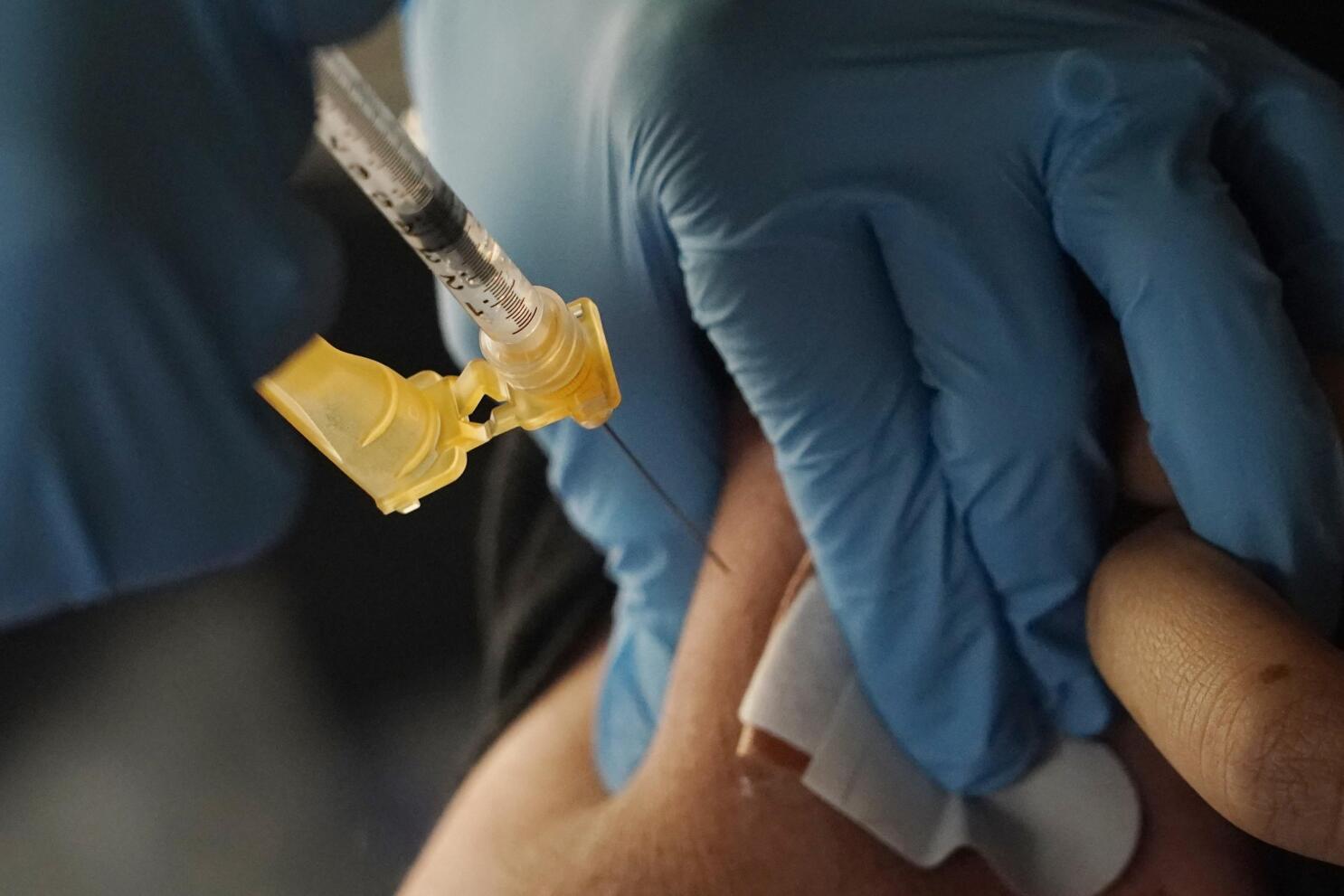 Extra COVID-19 booster now open to some high-risk Americans14 Jul 2023
Extra COVID-19 booster now open to some high-risk Americans14 Jul 2023
Tu pourrais aussi aimer
 Moule à embout de bûche PVC rayure14 Jul 2023
Moule à embout de bûche PVC rayure14 Jul 2023 Colle noire pour recoller vitre écran de téléphone & tablette, T700014 Jul 2023
Colle noire pour recoller vitre écran de téléphone & tablette, T700014 Jul 2023 Ravensburger GraviTrax PRO Giant Starter Set - Marble Run, for Children Age 8 + & GraviTrax Catapult14 Jul 2023
Ravensburger GraviTrax PRO Giant Starter Set - Marble Run, for Children Age 8 + & GraviTrax Catapult14 Jul 2023 /22189-large_default/crochet-pour-pous14 Jul 2023
/22189-large_default/crochet-pour-pous14 Jul 2023 Sifflet Fox 40® Pearl - Rose14 Jul 2023
Sifflet Fox 40® Pearl - Rose14 Jul 2023 Joyeux anniversaire 6e anniversaire bougie 6 ans' Magnet carré14 Jul 2023
Joyeux anniversaire 6e anniversaire bougie 6 ans' Magnet carré14 Jul 2023 Bosch Bleu 0601868109 GSR 12V-15 Perceuse-visseuse 12V 2.0Ah Li-Ion dans L-Boxx14 Jul 2023
Bosch Bleu 0601868109 GSR 12V-15 Perceuse-visseuse 12V 2.0Ah Li-Ion dans L-Boxx14 Jul 2023 Peluche Squeezamals 10 cm Vivid : King Jouet, Mini peluches Vivid - Peluches14 Jul 2023
Peluche Squeezamals 10 cm Vivid : King Jouet, Mini peluches Vivid - Peluches14 Jul 2023 Photo Albums for 4x6 photos Holds 500 | Premium Photo Album | Photo Album with 500 Picture Pockets | Acid Free Photo Album for Wedding, Birthday, Baby14 Jul 2023
Photo Albums for 4x6 photos Holds 500 | Premium Photo Album | Photo Album with 500 Picture Pockets | Acid Free Photo Album for Wedding, Birthday, Baby14 Jul 2023 Pâte à tartiner protéinée - Baouw bio14 Jul 2023
Pâte à tartiner protéinée - Baouw bio14 Jul 2023

