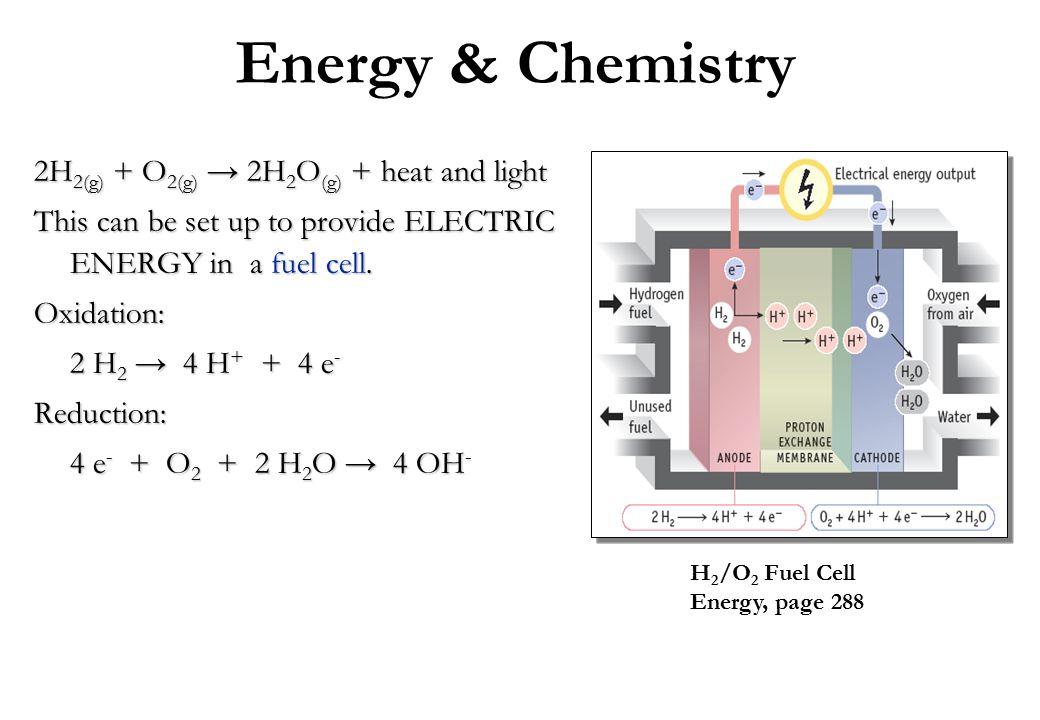
Energy & Chemistry 2H2(g) + O2(g) → 2H2O(g) + heat and light
Par un écrivain mystérieux
Last updated 18 mai 2024
Energy & Chemistry ENERGY is the capacity to do work or transfer heat. HEAT is the form of energy that flows between 2 objects because of their difference in temperature. Other forms of energy — light electrical kinetic and potential Positive and negative particles (ions) attract one another. Two atoms can bond As the particles attract they have a lower potential energy NaCl — composed of Na+ and Cl- ions.
Energy & Chemistry 2H2(g) + O2(g) → 2H2O(g) + heat and light
This can be set up to provide ELECTRIC ENERGY in a fuel cell. Oxidation: 2 H2 → 4 H+ + 4 e- Reduction: 4 e- + O2 + 2 H2O → 4 OH- H2/O2 Fuel Cell. Energy, page 288.
HEAT is the form of energy that flows between 2 objects because of their difference in temperature. Other forms of energy — light. electrical. kinetic and potential. Positive and negative particles (ions) attract one another. Two atoms can bond. As the particles attract they have a lower potential energy. NaCl — composed of Na+ and Cl- ions.
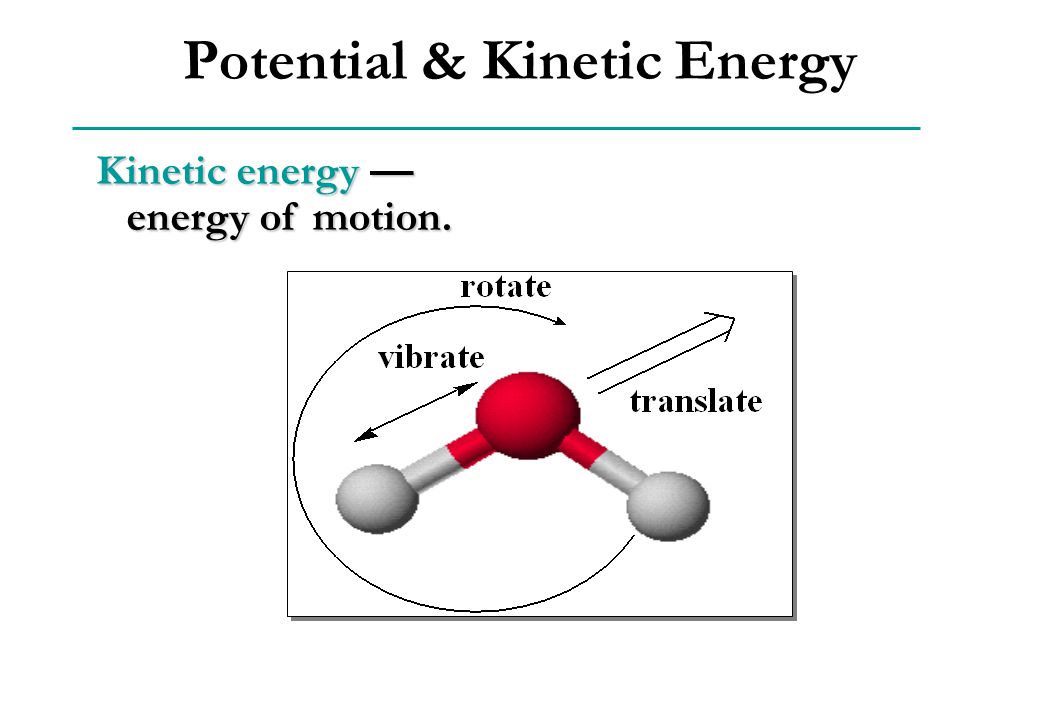
Kinetic energy — energy of motion.
PE + KE = Internal energy (E or U) Internal Energy of a chemical system depends on. number of particles. type of particles. temperature. The higher the T the higher the internal energy. So, use changes in T (∆T) to monitor changes in E (∆E).
∆T measures energy transferred. SYSTEM. The object under study. SURROUNDINGS. Everything outside the system.
Heat always transfer from hotter object to cooler one. EXOthermic: heat transfers from SYSTEM to SURROUNDINGS. T(system) goes down. T(surr) goes up.
Heat always transfers from hotter object to cooler one. ENDOthermic: heat transfers from SURROUNDINGS to the SYSTEM. T(system) goes up. T (surr) goes down.
Energy & Chemistry. All of thermodynamics depends on the law of. CONSERVATION OF ENERGY. The total energy is unchanged in a chemical reaction. If PE of products is less than reactants, the difference must be released as KE. Energy Change in Chemical Processes. Potential Energy of system dropped. Kinetic energy increased. Therefore, you often feel a Temperature increase.
The heat required to raise an object’s T by 1 ˚C. Which has the larger heat capacity
How much energy is transferred due to Temperature difference The heat (q) lost or gained is related to. a) sample mass. b) change in T and. c) specific heat capacity. Specific heat capacity. = heat lost or gained by substance (J) (mass, g) (T change, K)
Substance. Phase. cp J g-1 K-1. Cp J mol-1 K-1. Air (typical room conditionsA) gas Aluminium. solid Argon Copper Diamond Ethanol. liquid Gold Graphite Helium Hydrogen Iron Lithium Mercury Nitrogen Neon Oxygen Uranium Water. gas (100 °C) liquid (25 °C) solid (0 °C) All measurements are at 25 °C unless noted. Notable minimums and maximums are shown in maroon text. Aluminum. A Assuming an altitude of 194 meters above mean sea level (the world–wide median altitude of human habitation), an indoor temperature of 23 °C, a dewpoint of 9 °C (40.85% relative humidity), and 760 mm–Hg sea level–corrected barometric pressure (molar water vapor content = 1.16%).
If 25.0 g of Al cool from 310 oC to 37 oC, how many joules of heat energy are lost by the Al
q transferred = (sp. ht.)(mass)(∆T)
Drop into 225 g water at 21.0 ˚C. Water and metal come to 23.1 ˚C. What is the specific heat capacity of the metal
Note that T is constant as ice melts or water boils.
In general, reactions that transfer energy to their surroundings are product-favored. So, let us consider heat transfer in chemical processes.
heat energy transferred. ∆E = q + w. work done. by the. system. energy. change. Energy is conserved!
Exothermic reactions generate specific amounts of heat. This is because the potential energies of the products are lower than the potential energies of the reactants.
There are two basic ideas of importance for thermodynamic systems. Chemical systems tend toward a state of minimum potential energy. Chemical systems tend toward a state of maximum disorder. The first law is also known as the Law of Conservation of Energy. Energy is neither created nor destroyed in chemical reactions and physical changes.
(exothermic), -q. SYSTEM. ∆E = q + w. w transfer in. (+w) w transfer out. (-w)
Most chemical reactions occur at constant P, so. Heat transferred at constant P = qp. qp = ∆H where H = enthalpy. and so ∆E = ∆H + w (and w is usually small) ∆H = heat transferred at constant P ≈ ∆E. ∆H = change in heat content of the system. ∆H = Hfinal - Hinitial.
If Hfinal > Hinitial then ∆H is positive. Process is ENDOTHERMIC. If Hfinal < Hinitial then ∆H is negative. Process is EXOTHERMIC.
USING ENTHALPY. Consider the formation of water. H2(g) + 1/2 O2(g) → H2O(g) kJ. Exothermic reaction — heat is a product and ∆H = – kJ.
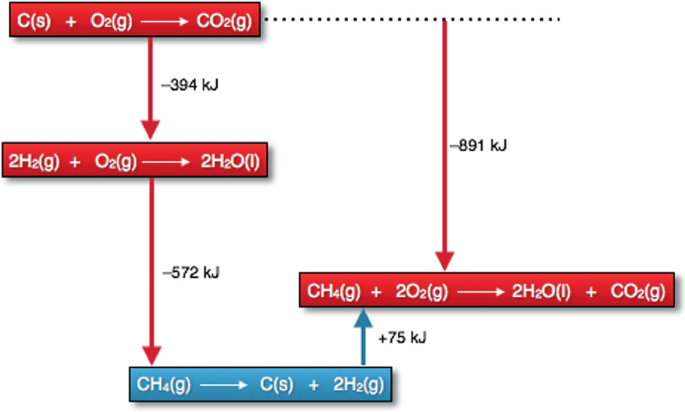
Liquid H2O. Making H2O from H2 involves two steps. H2(g) + 1/2 O2(g) → H2O(g) kJ. H2O(g) → H2O(l) + 44 kJ. H2(g) + 1/2 O2(g) → H2O(l) kJ. Example of HESS’S LAW— If a rxn. is the sum of 2 or more others, the net ∆H is the sum of the ∆H’s of the other rxns.
Forming H2O can occur in a single step or in a two steps. ∆Htotal is the same no matter which path is followed. Active Figure
Hess’s Law of Heat Summation, Hrxn = H1 +H2 +H , states that the enthalpy change for a reaction is the same whether it occurs by one step or by any (hypothetical) series of steps. Hess’s Law is true because H is a state function. If we know the following Ho’s.
Notice that reaction [1] has FeO and O2 as reactants and Fe2O3 as a product. Arrange reactions [2] and [3] so that they also have FeO and O2 as reactants and Fe2O3 as a product. Each reaction can be doubled, tripled, or multiplied by half, etc. The Ho values are also doubled, tripled, etc. If a reaction is reversed the sign of the Ho is changed.
For any chemical reaction at standard conditions, the standard enthalpy change is the sum of the standard molar enthalpies of formation of the products (each multiplied by its coefficient in the balanced chemical equation) minus the corresponding sum for the reactants.
calculate Ho for the reaction below.
Hess’s Law Use a little algebra and Hess’s Law to get the appropriate Hovalues
Notice that the energy change in moving from the top to the bottom is independent of pathway but the work required may not be! Some examples of state functions are: T (temperature), P (pressure), V (volume), E (change in energy), H (change in enthalpy – the transfer of heat), and S (entropy) Examples of non-state functions are: n (moles), q (heat), w (work) ∆H along one path = ∆H along another path. This equation is valid because ∆H is a STATE FUNCTION. These depend only on the state of the system and not how it got there. V, T, P, energy — and your bank account! Unlike V, T, and P, one cannot measure absolute H. Can only measure ∆H.
The properties of a system that depend only on the state of the system are called state functions. State functions are always written using capital letters. The value of a state function is independent of pathway. An analog to a state function is the energy required to climb a mountain taking two different paths. E1 = energy at the bottom of the mountain. E1 = mgh1. E2 = energy at the top of the mountain. E2 = mgh2. E = E2-E1 = mgh2 – mgh1 = mg(h)
Thermochemical standard state conditions. The thermochemical standard T = K. The thermochemical standard P = atm. Be careful not to confuse these values with STP. Thermochemical standard states of matter. For pure substances in their liquid or solid phase the standard state is the pure liquid or solid. For gases the standard state is the gas at 1.00 atm of pressure. For gaseous mixtures the partial pressure must be 1.00 atm. For aqueous solutions the standard state is 1.00 M concentration. ∆Hfo = standard molar enthalpy of formation. the enthalpy change when 1 mol of compound is formed from elements under standard conditions. See Table 6.2 and Appendix L.
2H2(g) + O2(g) → 2H2O(g) ∆H˚ = -484 kJ. H2O(g) → H2(g) + 1/2 O2(g) ∆H˚ = +242 kJ. H2(g) + 1/2 O2(g) → H2O(l) ∆H˚ = -286 kJ.
H2(g) + ½ O2(g) → H2O(g) ∆Hf˚ (H2O, g)= kJ/mol. C(s) + ½ O2(g) → CO(g) ∆Hf˚ of CO = kJ/mol. By definition, ∆Hfo = 0 for elements in their standard states. Use ∆H˚’s to calculate enthalpy change for. H2O(g) + C(graphite) → H2(g) + CO(g)
In general, when ALL enthalpies of formation are known, ∆Horxn = ∆Hfo (products) - ∆Hfo (reactants) Remember that ∆ always = final – initial.
Calculate the heat of combustion of methanol, i.e., ∆Horxn for. CH3OH(g) + 3/2 O2(g) → CO2(g) + 2 H2O(g) ∆Horxn = ∆Hfo (prod) - ∆Hfo (react)
Energy & Chemistry 2H2(g) + O2(g) → 2H2O(g) + heat and light
This can be set up to provide ELECTRIC ENERGY in a fuel cell. Oxidation: 2 H2 → 4 H+ + 4 e- Reduction: 4 e- + O2 + 2 H2O → 4 OH- H2/O2 Fuel Cell. Energy, page 288.
HEAT is the form of energy that flows between 2 objects because of their difference in temperature. Other forms of energy — light. electrical. kinetic and potential. Positive and negative particles (ions) attract one another. Two atoms can bond. As the particles attract they have a lower potential energy. NaCl — composed of Na+ and Cl- ions.
Kinetic energy — energy of motion.
PE + KE = Internal energy (E or U) Internal Energy of a chemical system depends on. number of particles. type of particles. temperature. The higher the T the higher the internal energy. So, use changes in T (∆T) to monitor changes in E (∆E).
∆T measures energy transferred. SYSTEM. The object under study. SURROUNDINGS. Everything outside the system.
Heat always transfer from hotter object to cooler one. EXOthermic: heat transfers from SYSTEM to SURROUNDINGS. T(system) goes down. T(surr) goes up.
Heat always transfers from hotter object to cooler one. ENDOthermic: heat transfers from SURROUNDINGS to the SYSTEM. T(system) goes up. T (surr) goes down.
Energy & Chemistry. All of thermodynamics depends on the law of. CONSERVATION OF ENERGY. The total energy is unchanged in a chemical reaction. If PE of products is less than reactants, the difference must be released as KE. Energy Change in Chemical Processes. Potential Energy of system dropped. Kinetic energy increased. Therefore, you often feel a Temperature increase.
The heat required to raise an object’s T by 1 ˚C. Which has the larger heat capacity
How much energy is transferred due to Temperature difference The heat (q) lost or gained is related to. a) sample mass. b) change in T and. c) specific heat capacity. Specific heat capacity. = heat lost or gained by substance (J) (mass, g) (T change, K)
Substance. Phase. cp J g-1 K-1. Cp J mol-1 K-1. Air (typical room conditionsA) gas Aluminium. solid Argon Copper Diamond Ethanol. liquid Gold Graphite Helium Hydrogen Iron Lithium Mercury Nitrogen Neon Oxygen Uranium Water. gas (100 °C) liquid (25 °C) solid (0 °C) All measurements are at 25 °C unless noted. Notable minimums and maximums are shown in maroon text. Aluminum. A Assuming an altitude of 194 meters above mean sea level (the world–wide median altitude of human habitation), an indoor temperature of 23 °C, a dewpoint of 9 °C (40.85% relative humidity), and 760 mm–Hg sea level–corrected barometric pressure (molar water vapor content = 1.16%).
If 25.0 g of Al cool from 310 oC to 37 oC, how many joules of heat energy are lost by the Al
q transferred = (sp. ht.)(mass)(∆T)
Drop into 225 g water at 21.0 ˚C. Water and metal come to 23.1 ˚C. What is the specific heat capacity of the metal
Note that T is constant as ice melts or water boils.
In general, reactions that transfer energy to their surroundings are product-favored. So, let us consider heat transfer in chemical processes.
heat energy transferred. ∆E = q + w. work done. by the. system. energy. change. Energy is conserved!
Exothermic reactions generate specific amounts of heat. This is because the potential energies of the products are lower than the potential energies of the reactants.
There are two basic ideas of importance for thermodynamic systems. Chemical systems tend toward a state of minimum potential energy. Chemical systems tend toward a state of maximum disorder. The first law is also known as the Law of Conservation of Energy. Energy is neither created nor destroyed in chemical reactions and physical changes.
(exothermic), -q. SYSTEM. ∆E = q + w. w transfer in. (+w) w transfer out. (-w)
Most chemical reactions occur at constant P, so. Heat transferred at constant P = qp. qp = ∆H where H = enthalpy. and so ∆E = ∆H + w (and w is usually small) ∆H = heat transferred at constant P ≈ ∆E. ∆H = change in heat content of the system. ∆H = Hfinal - Hinitial.
If Hfinal > Hinitial then ∆H is positive. Process is ENDOTHERMIC. If Hfinal < Hinitial then ∆H is negative. Process is EXOTHERMIC.
USING ENTHALPY. Consider the formation of water. H2(g) + 1/2 O2(g) → H2O(g) kJ. Exothermic reaction — heat is a product and ∆H = – kJ.
Liquid H2O. Making H2O from H2 involves two steps. H2(g) + 1/2 O2(g) → H2O(g) kJ. H2O(g) → H2O(l) + 44 kJ. H2(g) + 1/2 O2(g) → H2O(l) kJ. Example of HESS’S LAW— If a rxn. is the sum of 2 or more others, the net ∆H is the sum of the ∆H’s of the other rxns.
Forming H2O can occur in a single step or in a two steps. ∆Htotal is the same no matter which path is followed. Active Figure
Hess’s Law of Heat Summation, Hrxn = H1 +H2 +H , states that the enthalpy change for a reaction is the same whether it occurs by one step or by any (hypothetical) series of steps. Hess’s Law is true because H is a state function. If we know the following Ho’s.
Notice that reaction [1] has FeO and O2 as reactants and Fe2O3 as a product. Arrange reactions [2] and [3] so that they also have FeO and O2 as reactants and Fe2O3 as a product. Each reaction can be doubled, tripled, or multiplied by half, etc. The Ho values are also doubled, tripled, etc. If a reaction is reversed the sign of the Ho is changed.
For any chemical reaction at standard conditions, the standard enthalpy change is the sum of the standard molar enthalpies of formation of the products (each multiplied by its coefficient in the balanced chemical equation) minus the corresponding sum for the reactants.
calculate Ho for the reaction below.
Hess’s Law Use a little algebra and Hess’s Law to get the appropriate Hovalues
Notice that the energy change in moving from the top to the bottom is independent of pathway but the work required may not be! Some examples of state functions are: T (temperature), P (pressure), V (volume), E (change in energy), H (change in enthalpy – the transfer of heat), and S (entropy) Examples of non-state functions are: n (moles), q (heat), w (work) ∆H along one path = ∆H along another path. This equation is valid because ∆H is a STATE FUNCTION. These depend only on the state of the system and not how it got there. V, T, P, energy — and your bank account! Unlike V, T, and P, one cannot measure absolute H. Can only measure ∆H.
The properties of a system that depend only on the state of the system are called state functions. State functions are always written using capital letters. The value of a state function is independent of pathway. An analog to a state function is the energy required to climb a mountain taking two different paths. E1 = energy at the bottom of the mountain. E1 = mgh1. E2 = energy at the top of the mountain. E2 = mgh2. E = E2-E1 = mgh2 – mgh1 = mg(h)
Thermochemical standard state conditions. The thermochemical standard T = K. The thermochemical standard P = atm. Be careful not to confuse these values with STP. Thermochemical standard states of matter. For pure substances in their liquid or solid phase the standard state is the pure liquid or solid. For gases the standard state is the gas at 1.00 atm of pressure. For gaseous mixtures the partial pressure must be 1.00 atm. For aqueous solutions the standard state is 1.00 M concentration. ∆Hfo = standard molar enthalpy of formation. the enthalpy change when 1 mol of compound is formed from elements under standard conditions. See Table 6.2 and Appendix L.
2H2(g) + O2(g) → 2H2O(g) ∆H˚ = -484 kJ. H2O(g) → H2(g) + 1/2 O2(g) ∆H˚ = +242 kJ. H2(g) + 1/2 O2(g) → H2O(l) ∆H˚ = -286 kJ.
H2(g) + ½ O2(g) → H2O(g) ∆Hf˚ (H2O, g)= kJ/mol. C(s) + ½ O2(g) → CO(g) ∆Hf˚ of CO = kJ/mol. By definition, ∆Hfo = 0 for elements in their standard states. Use ∆H˚’s to calculate enthalpy change for. H2O(g) + C(graphite) → H2(g) + CO(g)
In general, when ALL enthalpies of formation are known, ∆Horxn = ∆Hfo (products) - ∆Hfo (reactants) Remember that ∆ always = final – initial.
Calculate the heat of combustion of methanol, i.e., ∆Horxn for. CH3OH(g) + 3/2 O2(g) → CO2(g) + 2 H2O(g) ∆Horxn = ∆Hfo (prod) - ∆Hfo (react)

CH104: Chapter 6 - Quantities in Chemical Reactions - Chemistry

Reaction of H2 with O2 in Excited Electronic States: Reaction Pathways and Rate Constants

The Magic Of Chemical Reaction And Its Types, by Rabiayousuf

PLEASE HELP!! Consider the following intermediate chemical equations. C(s) + O2(g) → CO2(g) AH, --393.5kJ

NCERT Exemplar Class 10 Science Solutions Chapter 1

Jamb Questions Chemistry FINAL 2, PDF, Reaction Rate
Energy SpringerLink
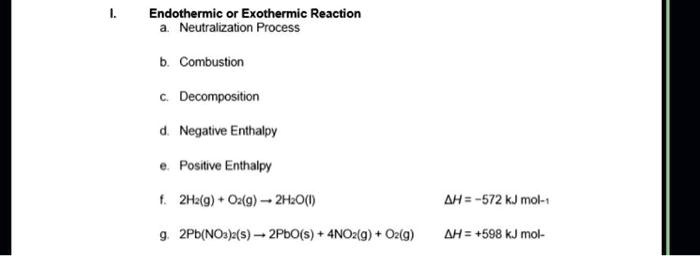
SOLVED: Endothermic or Exothermic Reaction Neutralization Process Combustion Decomposition Negative Enthalpy Positive Enthalpy 2H2O(l) 2H2(g) + O2(g) ΔH = 572 kJ mol-1 2Pb(NO3)2(s) - 2PbO(s) + 4NO2(g) + O2(g) ΔH = -598
PPT - IB Chemistry HL2 Thermochemistry (Energetics) PowerPoint Presentation - ID:4389758
Energy & Chemistry 2H2(g) + O2(g) → 2H2O(g) + heat and light - ppt video online download
Recommandé pour vous
 Gants chauffants confort + Batterie - G-HEAT - Loisir-Plein-Air14 Jul 2023
Gants chauffants confort + Batterie - G-HEAT - Loisir-Plein-Air14 Jul 2023 Gants chauffants CONFORT + Adulte G-HEAT14 Jul 2023
Gants chauffants CONFORT + Adulte G-HEAT14 Jul 2023 Run : G-Heat en mode esquimau glacé ! (test)14 Jul 2023
Run : G-Heat en mode esquimau glacé ! (test)14 Jul 2023 Chaussettes chauffantes G-Heat Outdoor V214 Jul 2023
Chaussettes chauffantes G-Heat Outdoor V214 Jul 2023 Gilet rafraichissant G-Heat EV05-GRE14 Jul 2023
Gilet rafraichissant G-Heat EV05-GRE14 Jul 2023 G-Heat : Le gilet rafraîchissant14 Jul 2023
G-Heat : Le gilet rafraîchissant14 Jul 2023 Olympia Heating and Cooling Services - G & G Heating14 Jul 2023
Olympia Heating and Cooling Services - G & G Heating14 Jul 2023 G-HEAT - Gants Chauffants Confort - Mixtes - Tactiles - Résistants14 Jul 2023
G-HEAT - Gants Chauffants Confort - Mixtes - Tactiles - Résistants14 Jul 2023 Veste softshell chauffante HV05 avec batterie 10000 mAh - G-HEAT14 Jul 2023
Veste softshell chauffante HV05 avec batterie 10000 mAh - G-HEAT14 Jul 2023 Equal heat is gives to two objects A and B of mass 1 g14 Jul 2023
Equal heat is gives to two objects A and B of mass 1 g14 Jul 2023
Tu pourrais aussi aimer
 Kit de raccordement Geberit pour WC suspendu longueur 18,5 cm avec14 Jul 2023
Kit de raccordement Geberit pour WC suspendu longueur 18,5 cm avec14 Jul 2023 PLAYMOBIL Pirate Ship Building Set : Toys & Games14 Jul 2023
PLAYMOBIL Pirate Ship Building Set : Toys & Games14 Jul 2023 Cache instruments de bord 1.9 TDI 2.0 MPI (km/h 0-260)14 Jul 2023
Cache instruments de bord 1.9 TDI 2.0 MPI (km/h 0-260)14 Jul 2023 Crayons de Cire Bic Kids Plastidecor 18 Couleurs14 Jul 2023
Crayons de Cire Bic Kids Plastidecor 18 Couleurs14 Jul 2023- Stölzle Lausitz Highlight Champagne Flutes with LED Lights, Set of14 Jul 2023
 Astuces pour organiser un barbecue pas cher et14 Jul 2023
Astuces pour organiser un barbecue pas cher et14 Jul 2023 Moccamaster by Technivorm KBGV Select 10-Cup Coffee Maker14 Jul 2023
Moccamaster by Technivorm KBGV Select 10-Cup Coffee Maker14 Jul 2023 Cardhu 12 Ans Scotch Whisky 40° Etui - Cardhu - Ecossais Whiskies & Bourbons Spiritueux - XO-Vin14 Jul 2023
Cardhu 12 Ans Scotch Whisky 40° Etui - Cardhu - Ecossais Whiskies & Bourbons Spiritueux - XO-Vin14 Jul 2023- 404 Billy - 0% (Terrain Glissant) on Vimeo14 Jul 2023
 Câble Ugreen Câble HDMI 4K 30 Hz 3D 18 10 m noir (HD104 10110)14 Jul 2023
Câble Ugreen Câble HDMI 4K 30 Hz 3D 18 10 m noir (HD104 10110)14 Jul 2023
