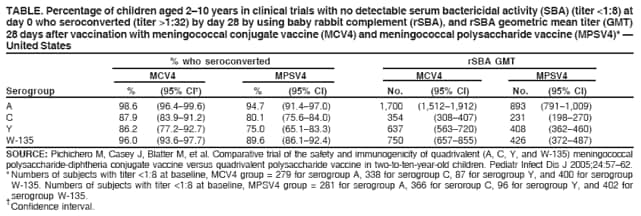
Report from the Advisory Committee on Immunization Practices (ACIP): Decision Not to Recommend Routine Vaccination of All Children Aged 2--10 Years with Quadrivalent Meningococcal Conjugate Vaccine (MCV4)
Par un écrivain mystérieux
Last updated 15 juin 2024

Immunization: Vaccine Updates Beyond COVID-19

An evaluation of emerging vaccines for childhood meningococcal disease, BMC Public Health
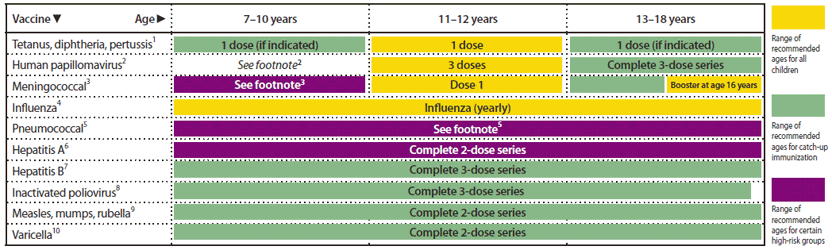
Recommended Immunization Schedules for Persons Aged 0 Through 18 Years — United States, 2012
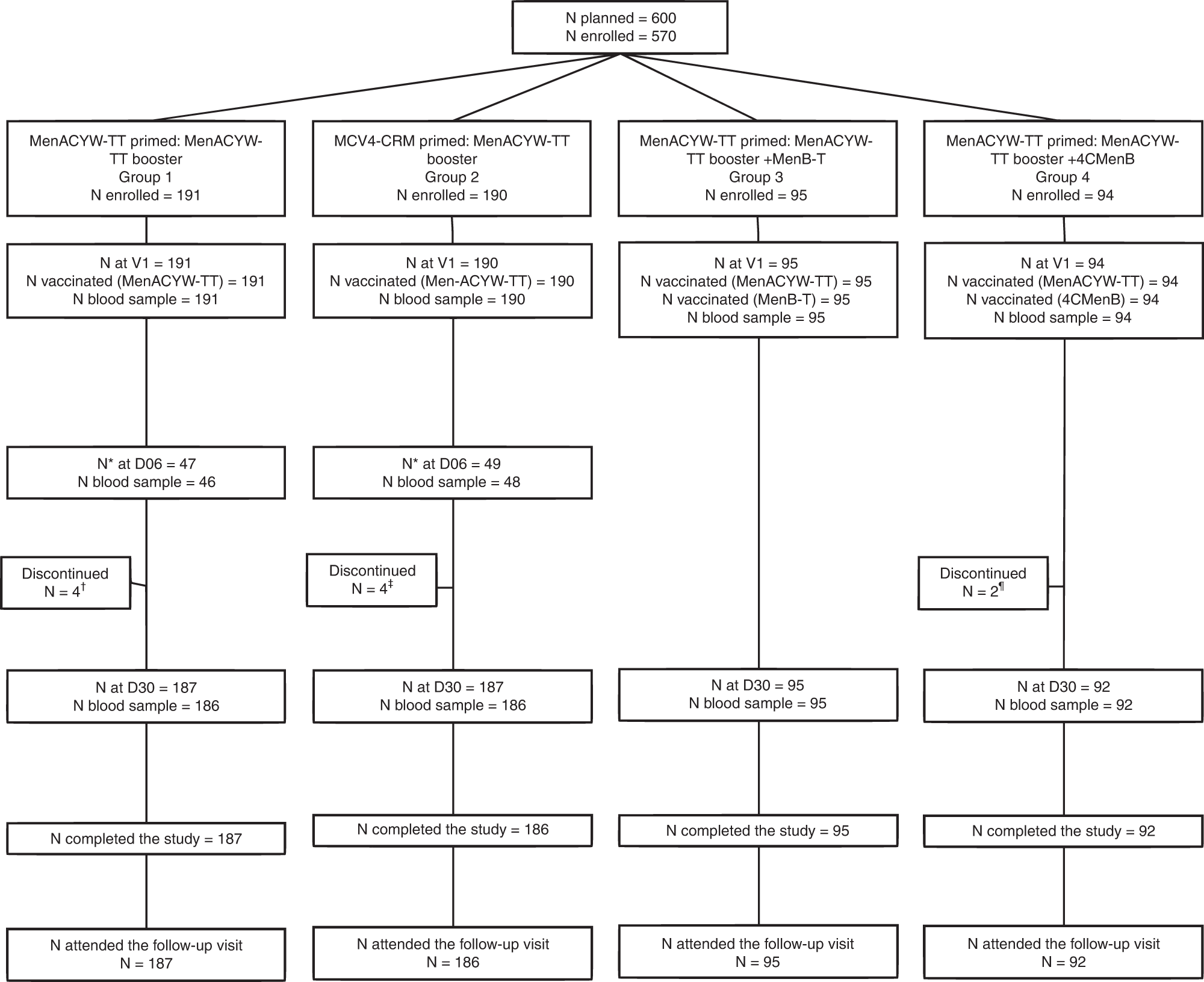
Quadrivalent meningococcal tetanus toxoid-conjugate booster vaccination in adolescents and adults: phase III randomized study
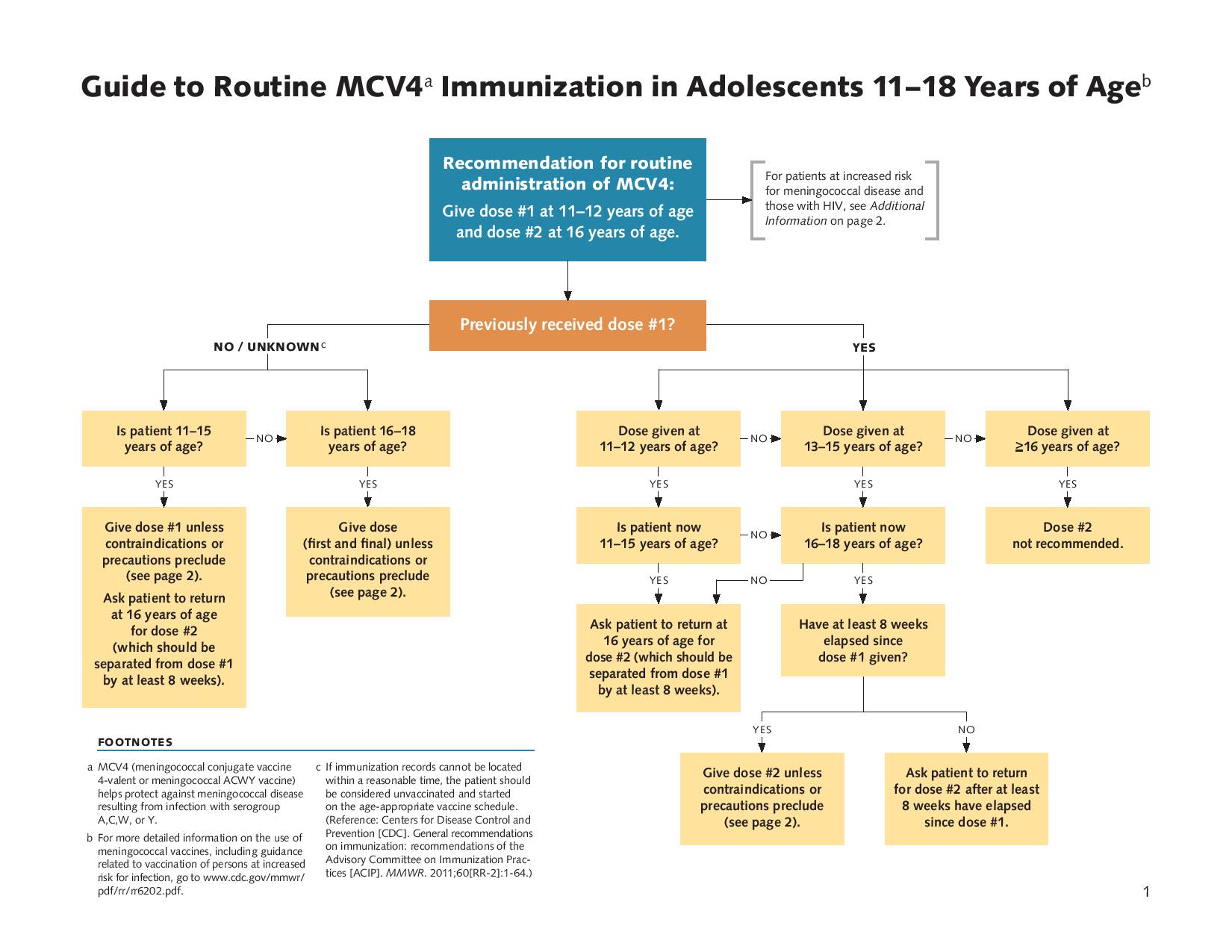
Meningococcal Vaccine Toolkit

Giving Teens a Boost? : Effects of Adolescent Meningococcal Vaccine Recommendations

A Half-Century of Prevention — The Advisory Committee on Immunization Practices
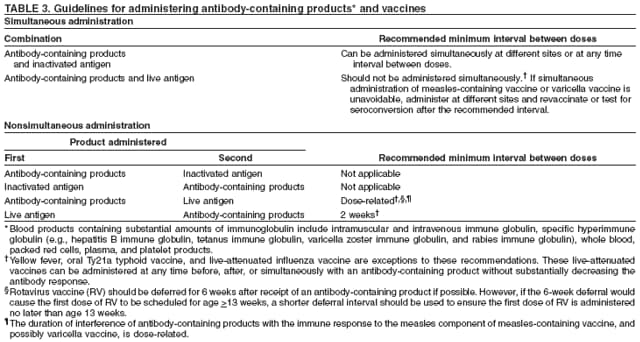
General Recommendations on Immunization
Recommendations of the Advisory Committee on Immunization Practices (ACIP)
ACIP Updates Recommendations for Meningococcal Vaccination

Update on Routine Childhood and Adolescent Immunizations

Pediatric Immunization: Meeting the Challenges of the 21st Century

Immunization Update - Advances in Pediatrics

Vaccination Recommendations for Pediatric Transplant Patients

Expert Perspectives on the Vaccination of Individuals Who Are at Increased Risk of Meningococcal Disease Due to Medical Conditions: A Podcast

Focus on the patient section.
Recommandé pour vous
 TOUR GAMING PREMIUM PERSONNALISÉE XXL MCV4 - Mars Gaming14 Jul 2023
TOUR GAMING PREMIUM PERSONNALISÉE XXL MCV4 - Mars Gaming14 Jul 2023 Missouri law requires meningitis vaccine for college students14 Jul 2023
Missouri law requires meningitis vaccine for college students14 Jul 2023 Meningitis: symptoms, causes, diagnosis & treatment + the MCV4 Vaccination14 Jul 2023
Meningitis: symptoms, causes, diagnosis & treatment + the MCV4 Vaccination14 Jul 2023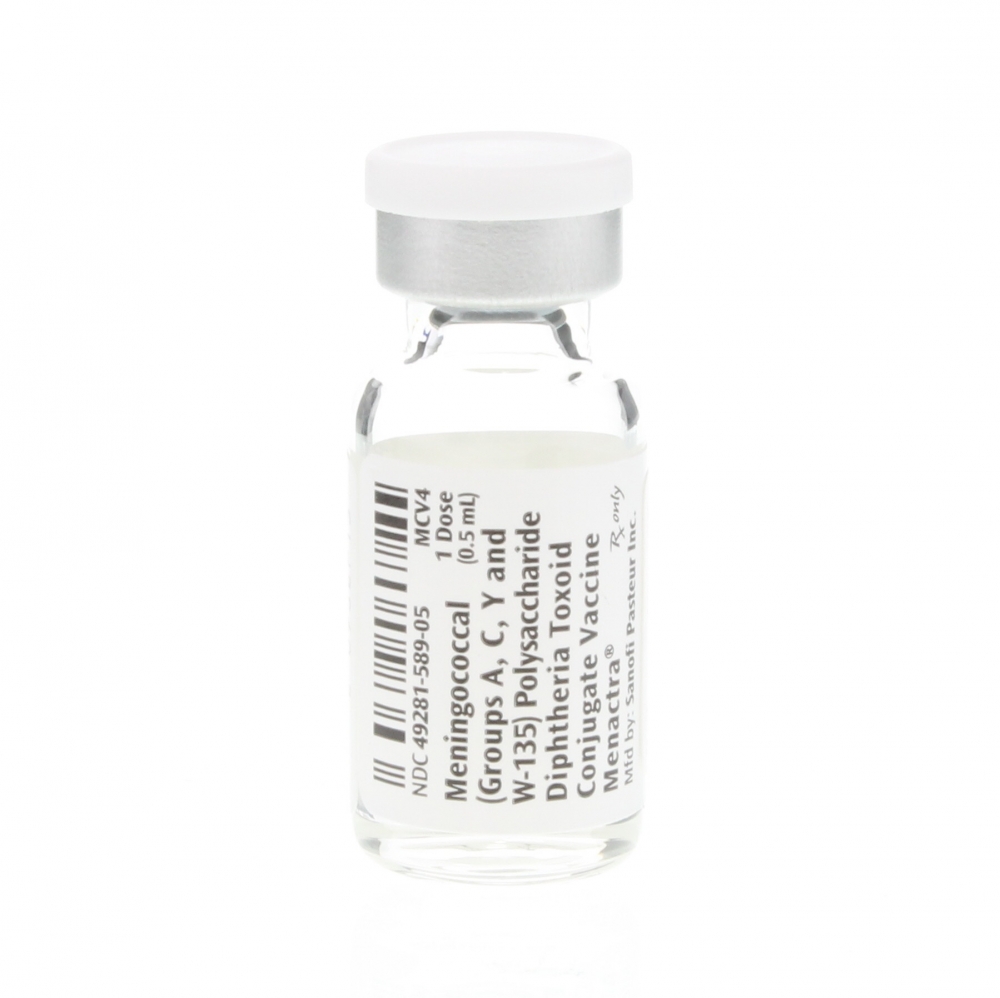 Menactra® 0.5ml SDV - Box/5: Clint Pharmaceuticals14 Jul 2023
Menactra® 0.5ml SDV - Box/5: Clint Pharmaceuticals14 Jul 2023 DIMAC MCV4 Angor -Intools14 Jul 2023
DIMAC MCV4 Angor -Intools14 Jul 2023 ECISD to hold vaccine clinics for Tdap and MCV414 Jul 2023
ECISD to hold vaccine clinics for Tdap and MCV414 Jul 2023 Doepfer - MCV4 MIDI-to-CV Interface – Noisebug14 Jul 2023
Doepfer - MCV4 MIDI-to-CV Interface – Noisebug14 Jul 2023 Doepfer MCV4 MIDI-CV Converter14 Jul 2023
Doepfer MCV4 MIDI-CV Converter14 Jul 2023 New law requires Ga. 11th-graders to get meningitis shot14 Jul 2023
New law requires Ga. 11th-graders to get meningitis shot14 Jul 2023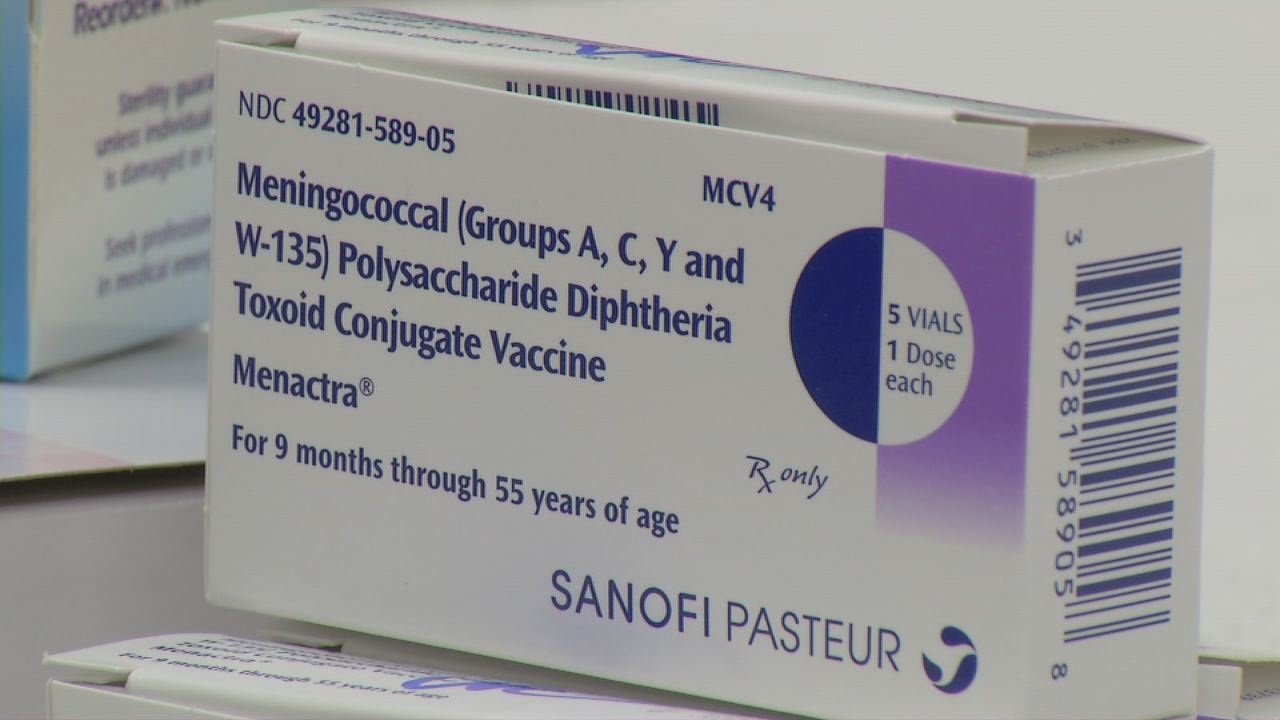 New immunization requirements for students in Iowa14 Jul 2023
New immunization requirements for students in Iowa14 Jul 2023
Tu pourrais aussi aimer
- LEGO Jurassic World 76944 T. rex Dinosaur Breakout14 Jul 2023
 Ordinateur portable éducatif bilingue Frozen - 124 activités14 Jul 2023
Ordinateur portable éducatif bilingue Frozen - 124 activités14 Jul 2023 High Density Firm Blue Foam Cut to Size14 Jul 2023
High Density Firm Blue Foam Cut to Size14 Jul 2023 Giantex Déshumidificateur Portable 25L/Jour pour Pièces 35-40m² - Déshumidificateur - Achat & prix14 Jul 2023
Giantex Déshumidificateur Portable 25L/Jour pour Pièces 35-40m² - Déshumidificateur - Achat & prix14 Jul 2023 FT430, Serre-câble durable et réutilisable pour suspension - Black Box14 Jul 2023
FT430, Serre-câble durable et réutilisable pour suspension - Black Box14 Jul 2023 Gilet Polaire Femme Melodie Kariban14 Jul 2023
Gilet Polaire Femme Melodie Kariban14 Jul 2023 10 Pièces Chaîne À Neige Universelle Chaîne De Pneu De - Temu Belgium14 Jul 2023
10 Pièces Chaîne À Neige Universelle Chaîne De Pneu De - Temu Belgium14 Jul 2023 Peut-on vraiment compter sur les podomètres?14 Jul 2023
Peut-on vraiment compter sur les podomètres?14 Jul 2023 T-shirt manches longues gris argent femme14 Jul 2023
T-shirt manches longues gris argent femme14 Jul 2023- Barre lumineuse Philips Hue Play White and Color Ambience (lot de deux) - Apple (CH)14 Jul 2023

