What is a Dossier in Regulatory Affairs
Par un écrivain mystérieux
Last updated 28 mai 2024

What is a dossier in Regulatory Affairs? Learn about the Common Technical Document (CTD) and all 5 Modules contained within.
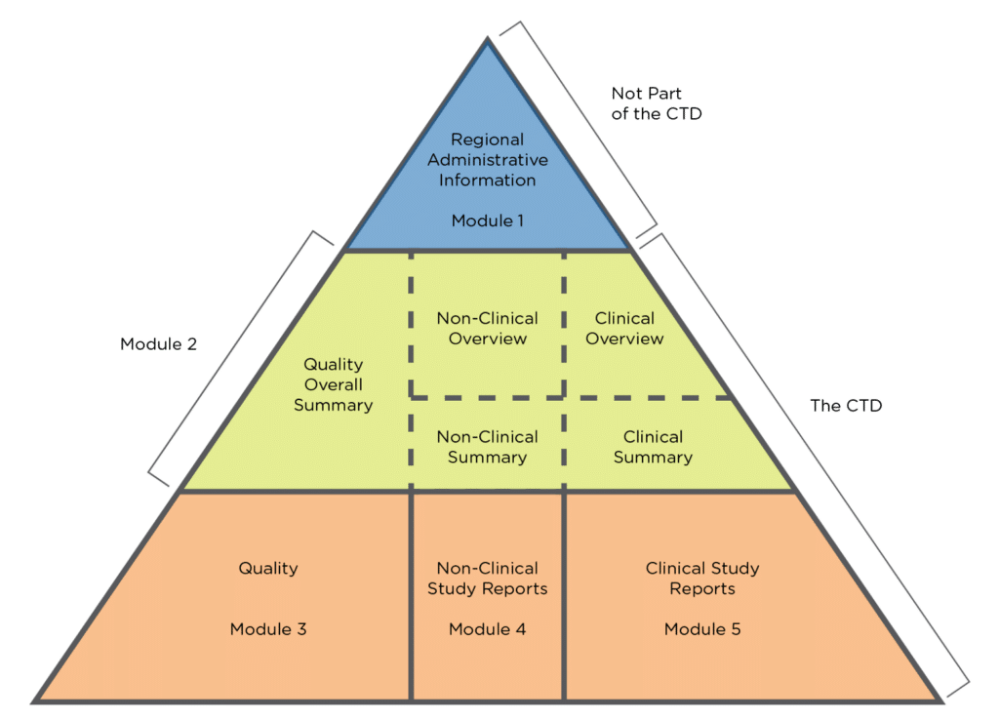
Understanding the Five Modules of the CTD Format in Regulatory Affairs for Pharmaceutical Submissions
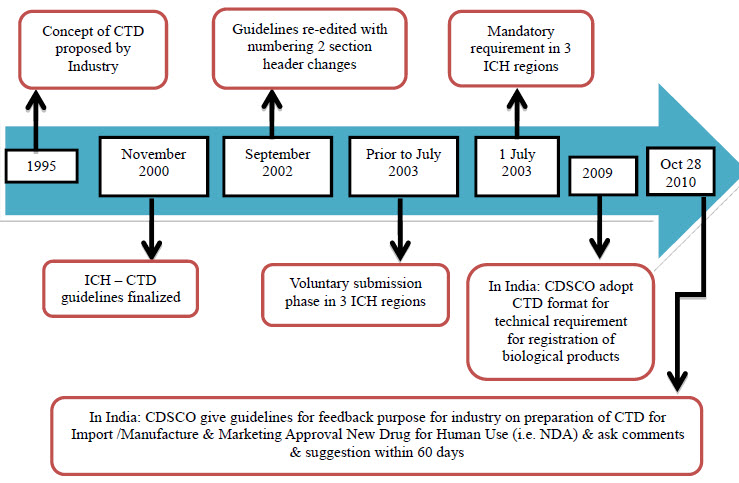
A STUDY OF PROCEDURES FOR DOSSIER PREPARATION AND THEIR MARKETING AUTHORISATION IN DIFFERENT COUNTRIES OF SELECTED DRUG(S)
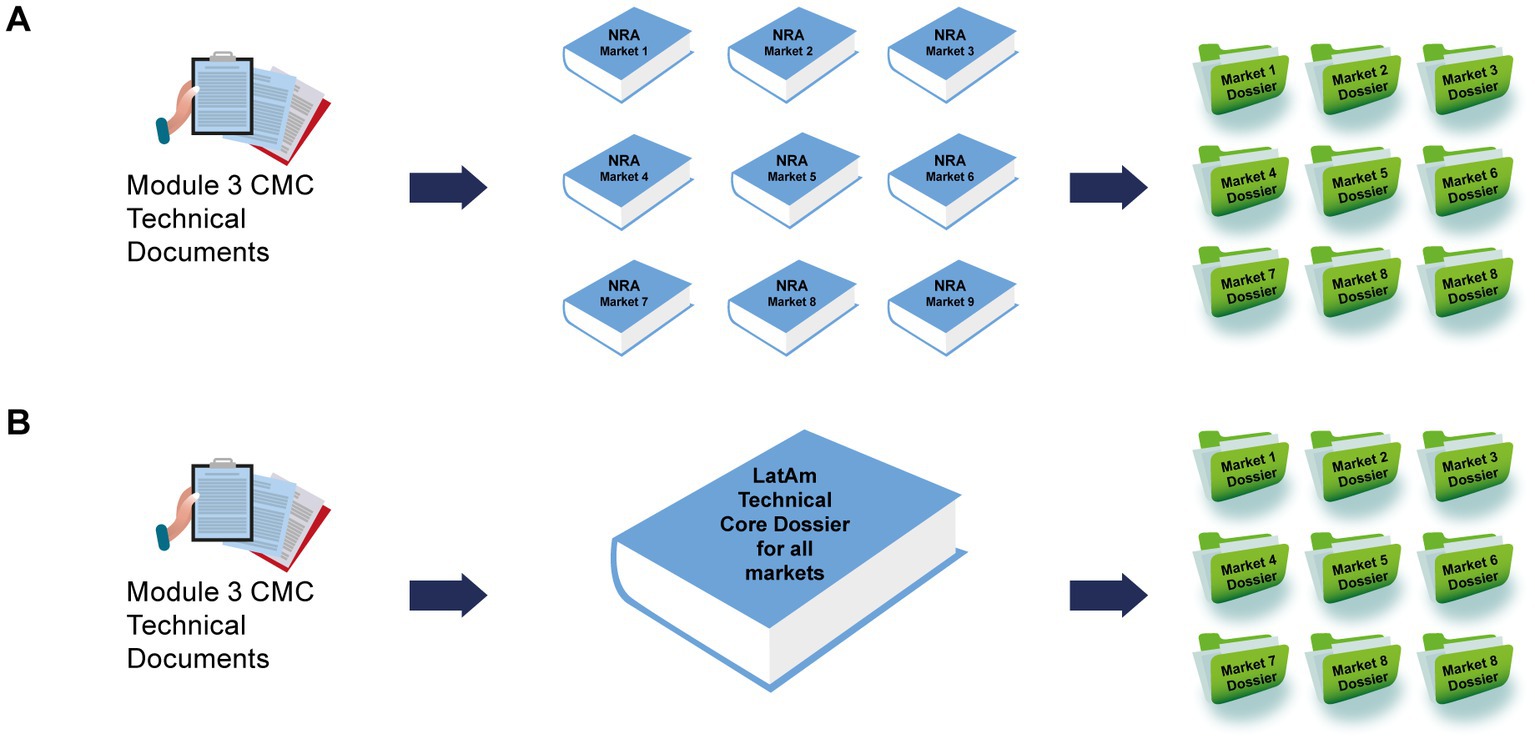
Frontiers Establishing a core dossier for multiple regulatory submissions: a case study in the Latin America region

Pharmaceutical Dossier submission

What Is A Dossier-Dossier Preparation As Per CTD Format, Regulatory Affairs

Regulatory Dossier Conversion, Preparation and Submission - Regulink

A STUDY OF PROCEDURES FOR DOSSIER PREPARATION AND THEIR MARKETING AUTHORISATION IN DIFFERENT COUNTRIES OF SELECTED DRUG(S)

Consulting Regulatory Affairs Services for Pharmaceutical companies

Regulatory Affairs Service Provider, eCTD Publishing, Submission
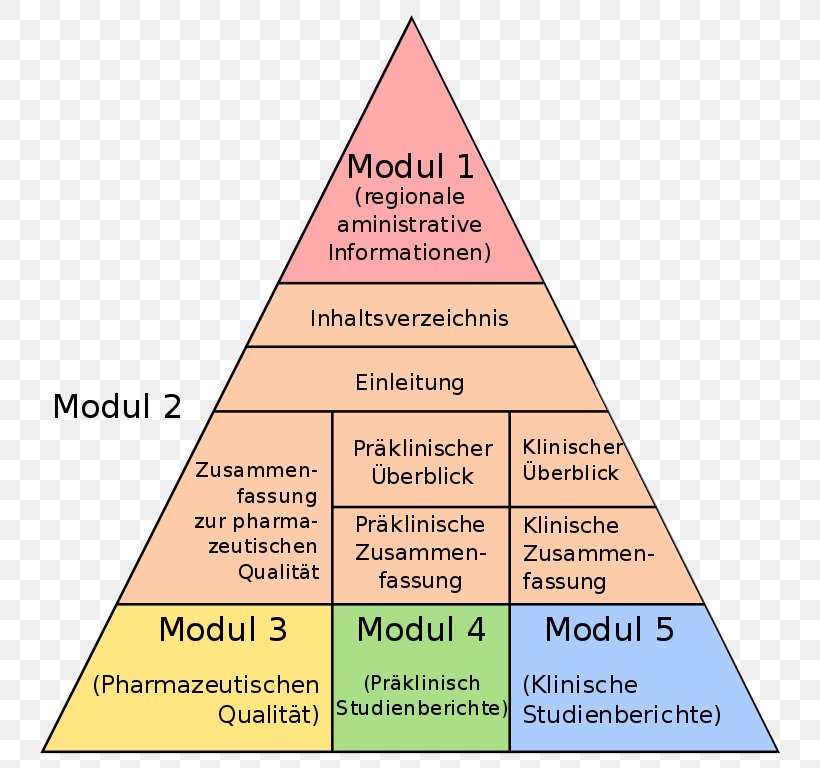
Electronic Common Technical Document Regulatory Affairs Triangle Pharmaceutical Drug, PNG, 770x768px, Regulatory Affairs, Area, Cone, Diagram

Regulatory Affairs - Q-support
Recommandé pour vous
 The dreaded dossier (and how we can help) - VINGT Paris14 Jul 2023
The dreaded dossier (and how we can help) - VINGT Paris14 Jul 2023 L'ouverture d'un dossier : ça se passe comment?14 Jul 2023
L'ouverture d'un dossier : ça se passe comment?14 Jul 2023 Dossier - Icônes fichiers et dossiers gratuites14 Jul 2023
Dossier - Icônes fichiers et dossiers gratuites14 Jul 2023 Consign 'Dossier' to the Round File - WSJ14 Jul 2023
Consign 'Dossier' to the Round File - WSJ14 Jul 2023 What Is a Dossier and How Should I Put One Together?14 Jul 2023
What Is a Dossier and How Should I Put One Together?14 Jul 2023 Premium Vector Company profile dossier template14 Jul 2023
Premium Vector Company profile dossier template14 Jul 2023 Constitution et gestion d'un dossier administratif14 Jul 2023
Constitution et gestion d'un dossier administratif14 Jul 2023 What Is Regulatory Dossier and What Does It Contain? - The14 Jul 2023
What Is Regulatory Dossier and What Does It Contain? - The14 Jul 2023 59,294 Dossier In Stock Photos, High-Res Pictures, and Images14 Jul 2023
59,294 Dossier In Stock Photos, High-Res Pictures, and Images14 Jul 2023 Creative Brand Dossier14 Jul 2023
Creative Brand Dossier14 Jul 2023
Tu pourrais aussi aimer
 PHYSIODOSE SERUM PHYSIOLOGIQUE AMPOULES 30 X 5 ML - Pharmacodel14 Jul 2023
PHYSIODOSE SERUM PHYSIOLOGIQUE AMPOULES 30 X 5 ML - Pharmacodel14 Jul 2023 Feuille de plexiglas transparent HD d'épaisseur 4 mm, panneau acrylique imperméable et résistant pour protection14 Jul 2023
Feuille de plexiglas transparent HD d'épaisseur 4 mm, panneau acrylique imperméable et résistant pour protection14 Jul 2023 Caterpillar présente une technologie de mini-pelle hydraulique facile à utiliser - 100 NE Adams St, Peoria, IL 61629, USA14 Jul 2023
Caterpillar présente une technologie de mini-pelle hydraulique facile à utiliser - 100 NE Adams St, Peoria, IL 61629, USA14 Jul 2023- LTV A-7D Corsair II > National Museum of the United States Air Force™ > Display14 Jul 2023
 Airtag Dog Collar Holder (2 Pack) - IPX8 Waterproof PETSTA Airtag Holder for Dog & Cat Collars, Ultra-Durable Lightweight PP Air Tag Dog Collar Holder14 Jul 2023
Airtag Dog Collar Holder (2 Pack) - IPX8 Waterproof PETSTA Airtag Holder for Dog & Cat Collars, Ultra-Durable Lightweight PP Air Tag Dog Collar Holder14 Jul 2023 Enceinte bluetooth - SRS-XB30 - Noir14 Jul 2023
Enceinte bluetooth - SRS-XB30 - Noir14 Jul 2023 Matelas à Mémoire de Forme Copenhague, Gel Refresh14 Jul 2023
Matelas à Mémoire de Forme Copenhague, Gel Refresh14 Jul 2023 Ballon Fête Blanc Gruilande Ballon Blanc Or pour Anniversaire Mariage Nuptiale Baptême Graduation Fête Décorations - Cdiscount Maison14 Jul 2023
Ballon Fête Blanc Gruilande Ballon Blanc Or pour Anniversaire Mariage Nuptiale Baptême Graduation Fête Décorations - Cdiscount Maison14 Jul 2023 Service de ménage à domicile à Albert – Services à la personne à14 Jul 2023
Service de ménage à domicile à Albert – Services à la personne à14 Jul 2023 Lime à ongles métal 17cm, Probeautic Institut14 Jul 2023
Lime à ongles métal 17cm, Probeautic Institut14 Jul 2023