How can graphite and diamond be so different if they are both
Par un écrivain mystérieux
Last updated 14 mai 2024


Finding and Making Diamonds Ask An Earth and Space Scientist

Moissanite vs. Diamond: Which Is the Best Engagement Ring?

SOLVED: Question 2. Diamond and graphite are both materials composed solely of carbon atoms. However; they exhibit dramatically different properties. Diamond is the hardest material known; whereas graphite is soft and shears
/images/uploads/diamonds.jpg)
How Do Diamonds Really Form? (Not From Coal!)
Both diamond and graphite are made from carbon. However, diamond is considered the hardest material, while graphite is brittle and slippery. What is this difference, from an atomic bonding view point?
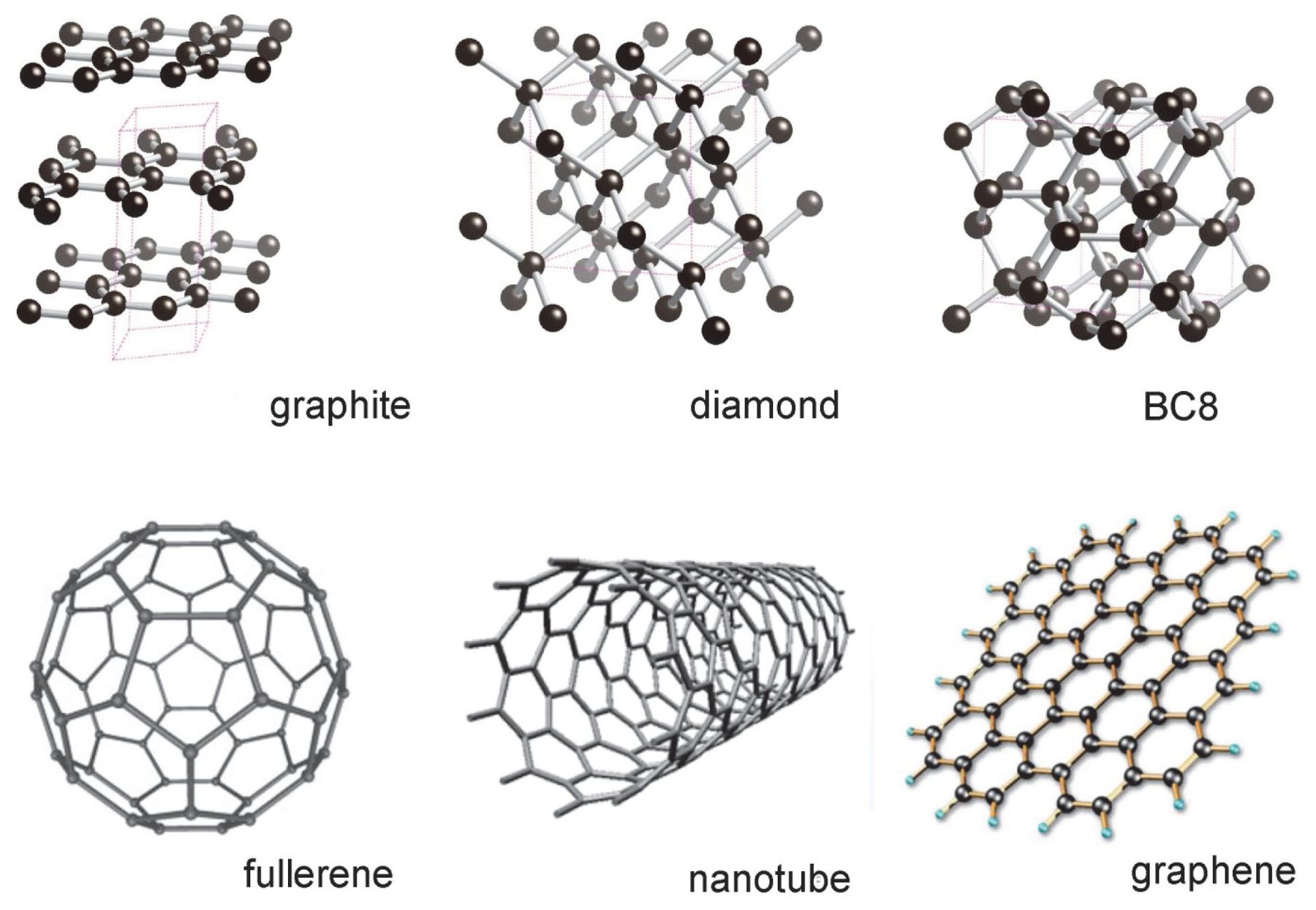
What are diamond and graphite in relation to carbon?

Can someone explain the difference between the two choices : r/Mcat
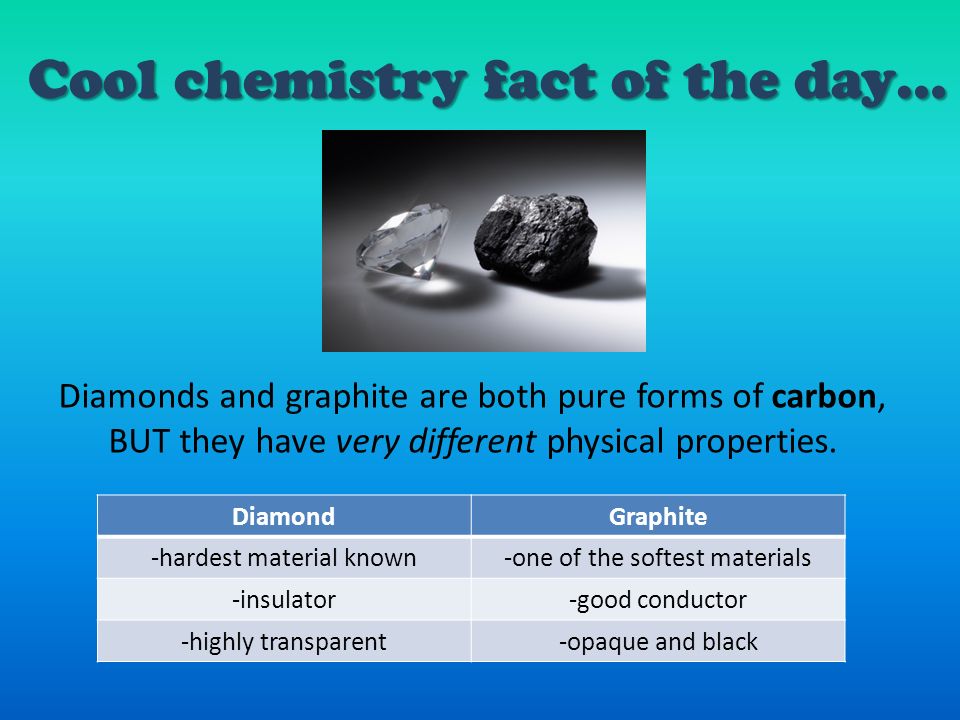
Cool chemistry fact of the day… Diamonds and graphite are both pure forms of carbon, BUT they have very different physical properties. DiamondGraphite. - ppt download

Why diamond and graphite have different physical properties but same chemical properties? What is the property called? - Quora
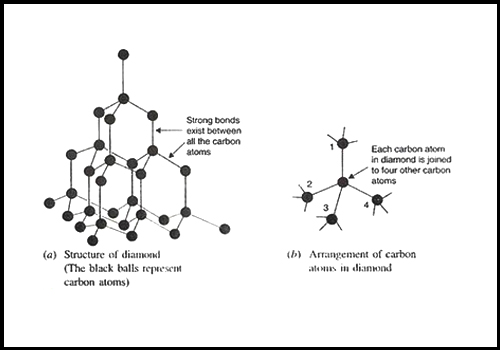
Describe why diamond is hard and graphite is soft?
Recommandé pour vous
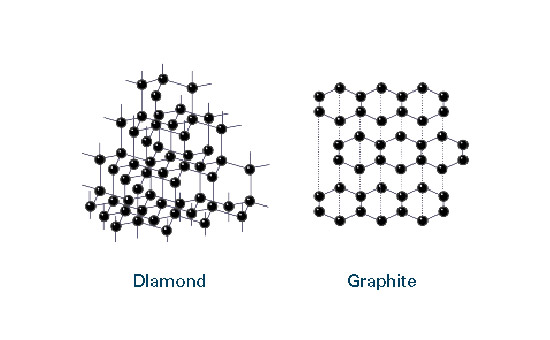 What is graphite? - ECGA14 Jul 2023
What is graphite? - ECGA14 Jul 2023 Graphite Stone - Virtues of the stones - Lithotherapy - Minerals14 Jul 2023
Graphite Stone - Virtues of the stones - Lithotherapy - Minerals14 Jul 2023 What is Graphite? - Mars Endüstri14 Jul 2023
What is Graphite? - Mars Endüstri14 Jul 2023 Graphite in pieces14 Jul 2023
Graphite in pieces14 Jul 2023 Explain the structure of Graphite.14 Jul 2023
Explain the structure of Graphite.14 Jul 2023 Natural graphite production expected to grow by 7.6% in 202114 Jul 2023
Natural graphite production expected to grow by 7.6% in 202114 Jul 2023- Graphite Aquarelle pencil, tin of 514 Jul 2023
 Graphite Concentrate - Mineral Commodities Ltd14 Jul 2023
Graphite Concentrate - Mineral Commodities Ltd14 Jul 2023 Increasing the Domestic Production of Graphite for Sustainability14 Jul 2023
Increasing the Domestic Production of Graphite for Sustainability14 Jul 2023 Choosing the Right Graphite Sketching & Drawing Pencil14 Jul 2023
Choosing the Right Graphite Sketching & Drawing Pencil14 Jul 2023
Tu pourrais aussi aimer
 Dessins en couleurs à imprimer La Petite Sirène numéro Fond d'écran petite sirène, Fond d'écran princesse disney, Ariel la petite sirène14 Jul 2023
Dessins en couleurs à imprimer La Petite Sirène numéro Fond d'écran petite sirène, Fond d'écran princesse disney, Ariel la petite sirène14 Jul 2023 Tapis de sol de voiture universel imprimé éléphant, tapis avant et14 Jul 2023
Tapis de sol de voiture universel imprimé éléphant, tapis avant et14 Jul 2023 Sacoche Cartable Homme Cuir14 Jul 2023
Sacoche Cartable Homme Cuir14 Jul 2023 Lucide TIRENO - Lanterne / lampadaire exterieur Extérieur - 1xE2714 Jul 2023
Lucide TIRENO - Lanterne / lampadaire exterieur Extérieur - 1xE2714 Jul 2023 Bibliothèque rotin - FLORES14 Jul 2023
Bibliothèque rotin - FLORES14 Jul 2023 Buy Crochet Pattern Simon Super Rabbit, Simon Super Rabbit Pattern, Crochet Pattern Super Rabbit, Simon Super Rabbit, Crochet Pattern Simon Online in India14 Jul 2023
Buy Crochet Pattern Simon Super Rabbit, Simon Super Rabbit Pattern, Crochet Pattern Super Rabbit, Simon Super Rabbit, Crochet Pattern Simon Online in India14 Jul 2023- Raccords pour tuyaux hydrauliques14 Jul 2023
 Cuisine & Electro - Les meilleurs bons plans14 Jul 2023
Cuisine & Electro - Les meilleurs bons plans14 Jul 2023 Comment placer des suspension mural en bois ?14 Jul 2023
Comment placer des suspension mural en bois ?14 Jul 2023 Flacon verre transparent 50 ml avec bouchon14 Jul 2023
Flacon verre transparent 50 ml avec bouchon14 Jul 2023

